Eggs and embryos: how aphids develop without sex
Aphids are parthenogenetic, that is they develop from unfertilised eggs, and they combine this with viviparity – they give birth to live young. Which evolved first, the parthenogenesis or the viviparity? That is a relatively easy question to answer. There are two groups of insects that are most closely related to true aphids, the adelgids and the phylloxerids, and they have a similar alternation of parthenogenetic and sexual generations. But in these two groups the parthenogenetic females as well as the sexual ones lay eggs. So we can be reasonably certain that the branch of the evolutionary tree leading to modern-day aphids diverged from that leading to these two groups at a time when parthenogenesis was already firmly established as part of the life system, yet before the process of developing embryos within the mother and giving birth to live young had evolved. Parthenogenesis came first, and then viviparity.
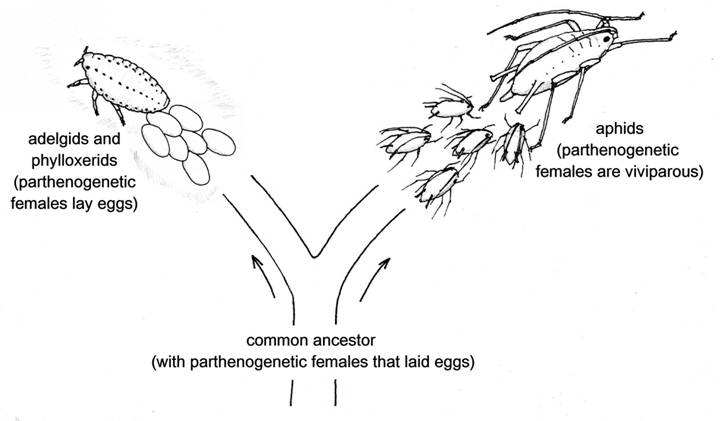
Fig. 1 Aphids’ closest relatives, the adelgids and phylloxerids, have parthenogenetic females that lay eggs.
The adelgids and phylloxerids are small groups, between them totalling about 125 species. The adelgids feed only on conifers – their common name is conifer woolly aphids – while the phylloxerids live on oaks and hickories, except for one species that is a notorious pest of grape vines. It seems likely that the far greater biological diversity of the true aphids, with about 4,700 species adapted to many different host plants and in numerous habitats, can be mainly attributed to their development of a method of combining the advantages of parthenogenesis with those of viviparity.
Many of the questions that biologists ask themselves are more problematic, as they tend to be about cause and effect. Why does this species look or behave like this, or why is it so common, or so rare? Often we try to find the answer by comparing it with other species that look or behave differently, but the differences are unlikely to be clear-cut. Species differ, not only in their genes but in the many ways that they interact with their environments, and rarely will it be possible to pin down the reason for any particular character or trait.
Biologists looking at parthenogenesis in aphids, however, find themselves in the happy situation of being able to make a direct comparison of the causes and consequences of parthenogenetic and sexual reproduction, not only in a single species, but in the same clone. Parthenogenetic, viviparous females can have the same genetic constitution – the same genotype – as sexual, egg-laying females, but differ greatly not only in the anatomy of their reproductive systems but in their external appearance and behaviour. It is a prime example of how epigenetic processes, acting on genes and their products to alter the ways in which they are expressed, can have drastic effects on the final outcome – the phenotype. In human terms, it would be like having genetically identical twin daughters and, when they grew up, finding that one gave birth to a rapid succession of babies while remaining a virgin, while the other waved her legs in the air to waft around scents that were irresistible to passing males, and after mating laid massive, baby-size, yolky eggs.
We can see exactly how far the reproductive organs of aphids have been modified for parthenogenesis, because we can compare them with those of individuals of the same species, or even the same clone, that mate and lay fertilised eggs. The ovaries of a sexual female aphid are very like those of other sap-sucking bugs in the order to which aphids belong – the Hemiptera. However, it is probably safe to assume that some readers will not be very well acquainted with the female genital organs of insects, so a little background information may not go amiss.
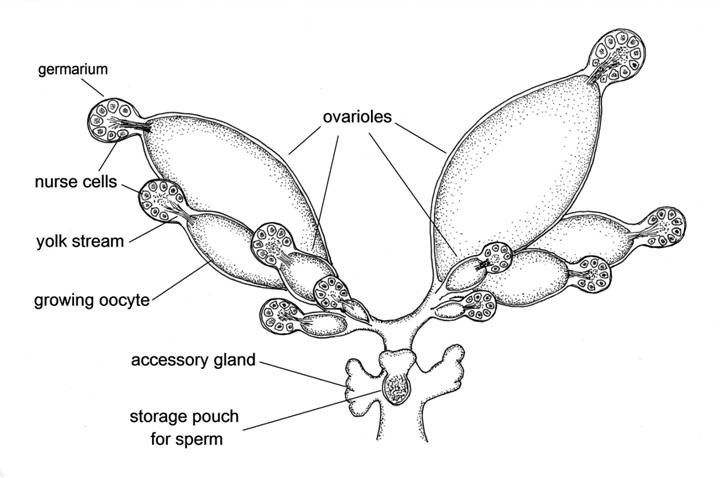
Fig. 2 Reproductive system of a sexual female aphid.
Female insects have a pair of ovaries as in vertebrates, but in insects each of these has several separate branches called ovarioles. The sexual female aphid illustrated here has five ovarioles in each ovary, and each of these has a spherical body at its tip, called a germarium. Each germarium when fully formed contains 32 germ cells, some of which are destined to become eggs, but at least half the cells in the germarium enlarge greatly in size to become nurse cells. The nurse cells are arranged in a sphere around a central core, and have the task of supplying a stream of yolk that passes in large quantities from the germarium into the developing egg. In order to fulfil this function the nuclei of the nurse cells have become polyploid; that is, they contain numerous sets of chromosomes, so that there are multiple copies of the DNA needed to produce the yolk proteins. In the illustration all the germaria have released one egg – more correctly termed an oocyte as it has not yet been fertilised – into the main part of the ovariole. The oocytes have all grown to different sizes, the first to be released from its germarium being the largest, and this will be the first to be fertilised and laid. When fully grown an oocyte is about 10,000 times larger than when it left the germarium. Each ovariole will produce at the most only one or two oocytes during the life of the sexual female aphid.
The ovaries meet to form a common oviduct, down which the oocytes pass one at a time as they become fully grown. At the lower end of the oviduct there is a pouch which receives and stores the sperm provided by the male during mating, and releases sperm to fertilise each oocyte as it passes. There are also a pair of accessory glands that secrete substances onto the surface of the egg after it has been fertilised and just before it is laid. These substances give the egg a protective coating, and also provide the glue that sticks it to the plant surface.
Now let us take a look at the reproductive organs of a typical parthenogenetic female aphid of the same species, or even the same clone:-
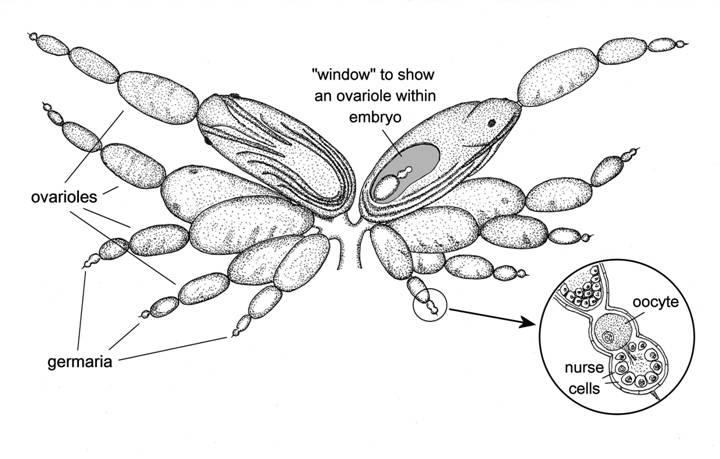
Fig. 3 Reproductive system of a parthenogenetic female aphid.
There are again five ovarioles, and each again has a germarium at its tip, but these germaria are of a different order of size – in fact only about one 500th of the volume of those of the sexual female. Yet the number of germ cells in the fully-formed germarium also starts out at 32, and at least half of these become nurse cells, the same as in the sexual, egg-producing female. The size difference is because when the nurse cells of the sexual female increase greatly in size, those of the parthenogenetic female don’t grow much, if at all, not needing multiple sets of chromosomes because they don’t have to provide anything like so much yolk. A group of germ cells at the base of the germarium are set aside to become oocytes, and as soon as the germarium is fully formed, no time is wasted before the first of these is released into the ovariole. Almost immediately, the cell divisions begin, the embryo starts to be formed, and in a very short time another oocyte leaves the germarium. Each ovariole becomes an embryo production line, slightly out of sync with all the other ovarioles, so that all the mother’s embryos are at slightly different stages of development, and reach the birth stage one at a time. The embryos pass through the common oviduct and are born “feet-first”, although without the complications of a breach birth because the legs are curled up in close proximity to the body. There is no pouch for storage of sperm as the mother does not mate, nor are there any accessory glands, because these too are surplus to requirements.

Fig. 4 An aphid being born.
There are some other crucial differences. The eggs of the sexual female aphid obviously cannot start to develop until after the mother has become adult and mated, and they have been fertilised and laid. But when the parthenogenetic mother aphid reaches adulthood she already has a string of developing embryos inside her, and is ready to give birth to the largest of these as soon as she becomes adult. In order for this to happen, her “pregnancy” has to start at an early age. In fact it starts at a very early age, just as soon as her germaria are fully formed, and that happens while she herself is still an embryo (see fig. 3). So the parthenogenetic mother aphid, when she becomes adult, not only has her first daughters ready to be born, but these daughters already have within them the embryos of her first granddaughters. This “telescoping” of generations into one another is the major reason for the phenomenal powers of multiplication of aphids, which will be examined in the next article.
A second difference which we have so far glossed over, but is if anything even more crucial, concerns the ability of the germ cells of parthenogenetic female aphids to develop without fertilisation. Fertilisation involves the fusion of the nucleus of the oocyte with that of a sperm, so that the fertilised egg has two sets of chromosomes, one from the mother and one from the father. The germ cells start out with two sets of chromosomes, so in the sexual female aphid as in any other sexual female there has to be a process by which the number of chromosome sets in the oocytes is reduced from two to one. This process is meiosis. Meiosis is one of those truly amazing developments early in the evolution of life on earth that is fundamental to all advanced organisms. But parthenogenetic female aphids have dispensed with meiosis, and the parthenogenetic oocytes retain both sets of the mother’s chromosomes, so that all the mother’s genes are passed on to her offspring.
So the parthenogenetic female aphid is a veritable baby factory, with ovarioles working like conveyor belts, churning out large numbers of offspring in the shortest possible time. That leaves an important question. How are the embryos provided with the nutrients that they need in order to grow so rapidly? Mammalian mothers have placentas to cater for the needs of developing embryos, but no placenta-like organs have ever been found in aphids. Developing oocytes and very young embryos nearest the germarium receive a stream of yolk supplied by the nurse cells, although this is nothing compared with the rivers of yolk that flows from the germaria of the sexual female to provision her eggs. But as the embryos pass further down the ovariole, and while they are still very small, they are cut off even from this limited supply of yolk. And yet there is no interruption to their growth.
The mystery deepens when we look at the poor diet that the mother has to cope with. Plant sap is a weak sugary solution with a very low concentration of the amino acids needed to make proteins. In order to obtain an adequate supply of nutrients, aphids have to imbibe a large quantity of sap and pass lots of fluid through their gut, excreting much of it as honeydew. Moreover plant sap almost completely lacks certain essential amino acids, that is, amino acids which animals cannot synthesise for themselves, and therefore have to acquire from an external source. With such an apparently poor diet, how does a parthenogenetic mother aphid provide enough nutrients to support her baby factory, and how do these nutrients get from the body cavity of the mother into her embryos?
We still have only partial answers to these questions, but we do know that they involve, to use the words of the yoghurt manufacturers, friendly bacteria. The technical term for such bacteria is endosymbiotic, meaning that they live inside the body of another organism, in a harmonious association that is of mutual benefit to both parties. Almost all aphids have the same type of friendly bacterium, called Buchnera, and this friendship has been going on for a remarkably long time. By studying the way in which the DNA of aphids and Buchnera have evolved together, molecular biologists have shown that this bacterium was probably present in the common ancestor of all living aphids (Baumann et al. 1997). So, one day more than 160 million years ago, an aphid ancestor acquired a bacterial infection, and the two have been living together ever since. Buchnera is related to the infamous Escherichia coli that inhabits the mammalian digestive system. E. coli usually lives in harmony with its host, but sometimes develops strains that have toxic effects. The long-established Buchnera-aphid relationship seems to be one of complete harmony, and in fact the two cannot live without each other. The aphid even provides specialised cells in its body cavity for Buchnera to inhabit, called bacteriocytes.
So what is it that the aphid gets in return for providing more than 160 million years of free accommodation? Biologists have been trying to answer this question by rearing aphids on an artificial diet which, unlike plant sap, can be made to provide all their nutritional requirements. They dosed some diet-reared pea aphids with an antibiotic that killed all their Buchnera, so that they were completely dependent on the diet for their essential amino acids. They then measured the effect on growth when one amino acid at a time was left out of the diet. The aphids that had been treated with the antibiotic virtually stopped growing when certain essential amino acids such as phenylalanine and threonine were omitted from their diet. The untreated aphids, however, which still had their Buchnera, could still grow normally (Akman Gündüz & Douglas 2009). So Buchnera makes up for the nutritional deficiencies in plant sap by manufacturing amino aphids that are essential to the aphid, but that it cannot make for itself.
As Buchnera is so vital to its existence, an aphid has to ensure that its bacteria are passed down from one generation to the next, no matter whether the offspring are produced parthenogenetically and viviparously, or sexually from fertilised eggs. In a sexual female aphid, Buchnera released from the bacteriocytes of the mother enter each egg when it is about half grown through pores in the wall of the follicle that surrounds it, and remain in a clump at one end of the egg until after it is fertilised and laid. However the embryos inside the parthenogenetic mother cannot wait, they need working bacteria right away to help to make their developing tissues. So at an early stage, when the embryo is still little more than a ball of cells, a large pore opens up and a great mass of bacteria flows into the embryo from the mother. The bacteria rapidly become packaged into bacteriocytes, with nuclei provided by the embryo, so that their work of synthesizing amino acids and other molecules essential for the development of the embryos can commence (Miura et al. 2003). They also rapidly proliferate, so that the mother aphid soon has many more bacteria within her embryos than were originally present in her own body cavity.
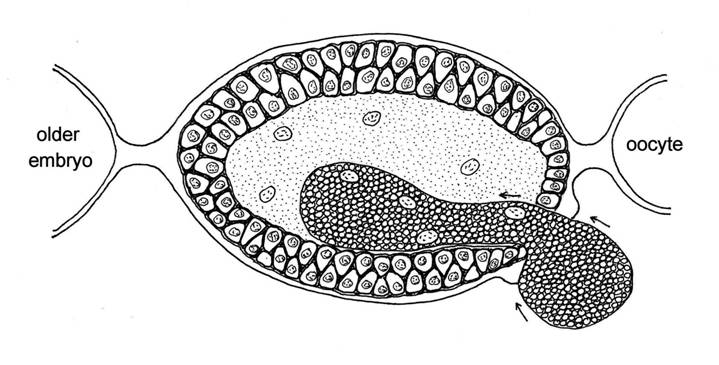
Fig. 5 A mass of bacteria (Buchnera) entering a young pea aphid embryo from the body cavity of the mother.
Biologists have now developed techniques to study the metabolism of aphid embryos separately from that of their mothers, and to relate this to the genes expressed by bacteria in embryos of different ages. It seems that the Buchnera genes involved in synthesis of several essential amino acids are very active in young embryos, as also are those responsible for the manufacture of riboflavin (vitamin B2). Another group of active genes in Buchnera at this stage are those involved in producing the flagellar apparatus, the amazing propeller-like microstructure that free-living bacteria use to move themselves around. Buchnera is like other bacteria that live within the cells of organisms, in no longer having need of a “propeller”, but some components of the flagellar structure are still present, and are thought to be involved in the transport of proteins across cell boundaries (Bermingham et al. 2009). This all helps to confirm that Buchnera plays an important part in the rapid growth and development of parthenogenetic aphid embryos. However, there is still lots that we don’t know about the way in which the metabolic activities of the two organisms interact.
It also leaves open the very big question of how the mother aphid provides all the nutrients that the embryos need in order to grow so quickly. We have already noted that aphids have nothing equivalent to the mammalian placenta, and the ovariole sheath that surrounds the developing embryos seems to be of simple structure, a single layer of cells. Radioactively-labelled essential amino acids injected into the mother aphid’s body cavity are taken up by embryos at different rates, indicating that they do not simply pass unimpeded through pores in the ovariole sheath. There is clearly some process of selective uptake going on (Bermingham & Wilkinson 2009). Yet the passage of some substances from mother to embryo can be very rapid. Tritium-labelled thymidine can be incorporated into the DNA of the embryo’s cell nuclei within 30 minutes of injection into the mother (Blackman 1974). It is rather surprising that we still know so little about such a key factor contributing to the great powers of multiplication of aphids.
References:
Akman Gündüz, E. & Douglas, A.E. (2009) Symbiotic bacteria enable insect to use a nutritionally inadequate diet. Proceedings of the Royal Society B 276: 987-991.
Baumann, P., Moran, N.A. & Baumann, L. (1997) The evolution and genetics of aphid endosymbionts. BioScience 47: 12-20.
Bermingham, J., Rabatel, A., Calevro, F., Viñuelas, J., Fevay, G., Charles, H. Douglas, A. & Wilkinson, T. (2009) Impact of host developmental age on the transcriptome of the symbiotic bacterium Buchnera aphidicola in the pea aphid (Acyrthosiphon pisum). Applied and Environmental Microbiology 75: 7294-7297.
Bermingham, J. & Wilkinson, T.L. (2009) Embryo nutrition in parthenogenetic viviparous aphids. Physiological Entomology 34: 103-109.
Blackman, R. L. (1974) Incorporation of thymidine into the chromosomes of aphid (Myzus persicae) embryos. Experientia 30: 1136-1137.
Miura, T., Braendle, C., Shingleton, A., Sisk, G., Kambhampati, S. & Stern, D.L. (2003) A comparison of parthenogenetic and sexual embryogenesis of the pea aphid Acyrthosiphon pisum (Hemiptera: Aphidoidea). Journal of Experimental Zoology (Molecular Development & Evolution) 295B: 59-81.